1. INTRODUCTION
An incorrect perception that most people working in a technical environment has, is that all Polymers are Plastics , and vice versa. This is most definitely not the case and the aim of this article is to clarify the difference between Polymers and Plastics. Both terms are furthermore used interchangeably, but in reality , these materials are vastly different.
The main differences between Polymers and Plastics are based on their Chemical Make-up, Physical Characteristics, and Practical Applications.
Plastics, on the other hand, are a class of Polymers made up of long, branched molecules, or macromolecules.
It is more often than not , prudent to obtain expert advice, about the choice or application of either the Polymer or Plastic to be applied. The application of Polymers and Plastics is Gennisi Solutions’ area of expertise –feel free to contact us for a free assessment and resultant proposal.
2. POLYMERS
Polymers can either be naturally occurring; termed Organic Polymers, or man-made i.e. Synthetic Polymers. Wool, cotton, and wood are all examples of Organic (naturally occurring) Polymers, while man-made polymers can range from Semi-Organic to totally Synthetic Polymers.
Before we proceed into the detail of Polymers , let’s look at the building blocks of Polymers.
Monomers
Monomers are simple molecules with two or more binding sites through which it forms linkages with other monomer molecules, to form macromolecules.
A Monomer is a single atom, small molecule, or molecular fragment that, when bonded together with identical or similar types of monomers, form a larger macromolecule called a Polymer.
An example of a Monomer structure is;
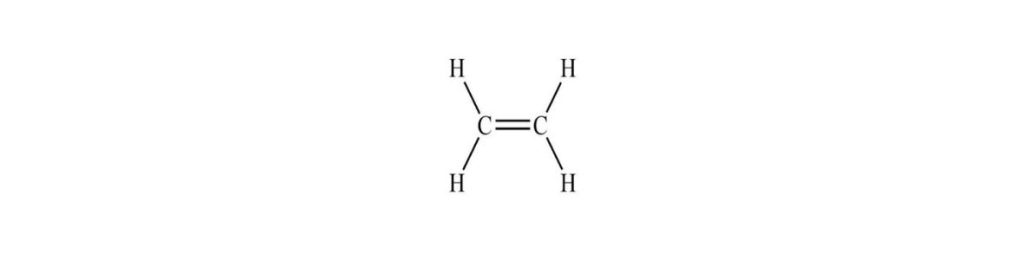
Examples of Monomers are ;
- Ethylene
- Propylene
- Styrene
- Phenol
- Acetonitrile
- Formaldehyde
- Ethylene glycol
- Vinyl chloride
Polymers
A polymer is a large molecule, or macromolecule, composed of small, repeating singular molecular structural units called monomers. The repeating molecular units are joined together chemically through covalent bonds (a covalent bond occurs where two atoms share an electron).
The word Polymer comes from the Greek “poly”(many) and “meros” (part). As with monomers, a polymer may be a natural (biopolymer) or a synthetic macromolecule (manufactured or synthesized), comprised of repeating units.
A polymer can be envisioned as a chain , with each of the links a monomer. Those monomers can be simple — just an atom or two or three — or they might be complicated ring-shaped structures containing a dozen or more atoms. In an artificial polymer, each of the chain’s links will often be identical to its neighbour’s.
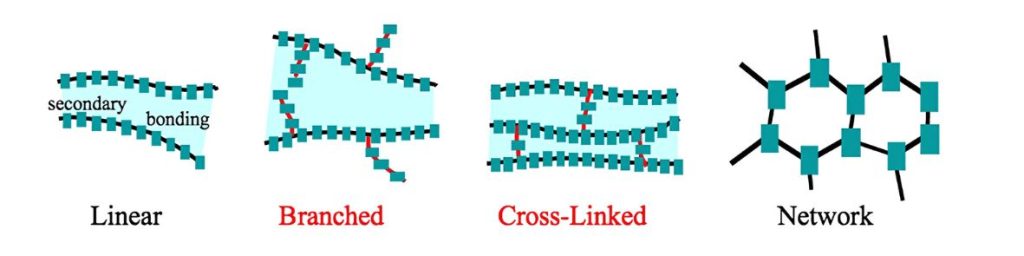
An example of a polymer structure is as follow;

There are four basic polymer structures, shown in the figures below. In practice, some polymers might contain a mixture of the various basic structures. The four basic polymer structures are linear, branched, crosslinked, and networked.
Examples of Polymers are;
- Nylon
- Polyethylene
- Polyester
- Teflon
- Epoxy
In Material Sciences, the terms ‘polymer’ and ‘plastic’ are often, and incorrectly so, used interchangeably; Polymers are a much larger class of molecules that includes plastics and have a broad range of properties that can perform various functions, thus be applied in a very wide variety of applications.
3. APPLICATION OF POLYMERS.
The Polymer to be applied in a particular application will demand review and clarification of the following;
i. The Structure of the Polymer
ii. The Properties of the Polymer
iii. The Application of the Polymer
3.1 The structure of the Polymer
Polymers have fundamental qualities and characteristics that are defined by its molecular structure. The following structural characteristics of a polymer must be considered when attempting to place it into a particular category: The polymer molecule consists of a “skeleton”. The following structural characteristics of the polymer’s “skeleton” must be considered when attempting to place it into a certain category , is the skeleton;
- Linear structure.
- Branched chain structure.
- Network structure.
The use of monomers; A monomer is defined as a simple molecule with two or more bonding sites through which it forms linkages with other monomer molecules, to form a macromolecule. To properly categorize a material, one must know its monomer composition, including the types and amounts of monomers that make up the polymer chain. Monomers are classified based on its origin (Organic or Naturally Occurring monomers), or on its synthesis (Manufactured or Synthesized monomers).
Examples of Organic Monomers are;
- Starches are polymers of monomer glucose
- Cellulose is also a polymer of monomer glucose. It is made of glucose produced during the photosynthesis in plants
- Protein is obtained as a result of polymerization of monomer amino acids
- Synthetic polymers are man-made. Examples hereof are polyethylene , polystyrene, PVC, Nylon and Dacron.
The molecular structure of a polymer can be described at various length scales, from the sub-nm length scale up to the macroscopic ones.
To synchronize a Polymer correctly, there is a hierarchy of structures in which each stage provides the foundation for the next one. The starting tip of a polymer’s structure is its component monomer’s identity. Thereafter the microstructure essentially represents the monomer sorting and arrangement within the Polymer until a single chain is formed.
The microstructure plays a crucial role in defining the possibility of a polymer to configure phases, with different arrangements, resulting in the final Polymer.
The molecular structure of a polymer defines the fundamental properties of the Polymeric material. The following are the points crucial in classifying a particular polymer material;
- Molecular bonds: The polymer structure heavily depends on how the monomers are linked or if there are no cross-branching bonds between polymer chains.
- Monomer configuration: To better classify material, knowing which monomers make up the polymer chain, how many of each, and the nature of those monomers will play a vital role.
- Chain attributes: The average length and weight of the chains in a polymer help determine the degree of polymerization and the molecular configuration of the polymer.
- Polymerization method: The methods used to join the monomers into polymers decide the structure of the polymer, regardless of whether it is natural or synthetic polymerization, through using heat, condensation, or chemicals. Addition or Chain polymers – this involves the repeated addition of monomers to form the Polymer Chain. The monomer and the resultant chain growth of the polymerization compound is tabulated below;
Monomers and the chain growth polymerization compounds are as follow;

The degree of polymerization and the molecular shape of a polymer can be inferred from the average chain length and weight of the polymer.
Connections between individual molecules defines the Polymers as well; whether or not there are cross-branching bonds between polymer chains and the ways in which the monomers are connected together define the polymer’s structure.
The process of polymerization furthermore too defines the Polymer; whether through a naturally occurring process or by synthetic polymerization via heat, chemicals, or condensation, the structure of the polymer is determined by the method by which the monomers are joined into polymers.
It is possible to divide synthetic polymers into three distinct groups:
i. Elastomers; for example, rubber, are elastic materials characterized by weak molecular connections that allow for a high degree of deformation.
ii. Polymer fibers; the molecular bonds in polymer chains used to make polymer fibers are far more robust than those in elastomers. Fibers, which can be made from either natural or synthetic materials, are more rigid and less elastic than elastomers.
iii. Thermoplastics ; these are distinguished from fibers and elastomers by their greater rigidity and the ability to maintain their molecular structure when heated. Thermoplastics are superior to combustibles when it comes to being molded and formed since they melt when heated to their melting point.
The classification of synthetic polymers is dependent on their basic structure, physical properties, and applications.
There are thousands of polymers available today; therefore, it’s crucial to comprehend their characteristics and potential applications properly before a final Polymer is chosen.
Polymers can be further categorized by their final physical form and exist in a wide range of shapes, sizes, and colors. The following are some distinguishing features:
- Density
- Conditions of heat
- Structure of crystals
- Hardness
- Tensile strength
- Machinability
- Formability
- Solubility , etc.
The application of polymers is defined, by the Properties of the Polymer. The properties , in turn can be divided into two categories; the Physical and Chemical. These properties in turn define the Applications of the Polymers.
i. Physical Properties
Polymers don’t melt; they change state from crystalline to semi-crystalline.
As the chain length and cross-linking increase, the tensile strength of the polymer also increases.
ii. Chemical Properties
Polymers are enabled with hydrogen bonding and ionic bonding resulting in superior cross-linked strength.
Polymers with Van der Waals forces linking chains are weak, giving a polymer a low melting point. Van der Waals forces is a general term used to define the attraction of intermolecular forces between molecules.
iii. Applications
As stated above, Polymers are created by a polymerization process and the number of the various polymers that can be created through the polymerization process is practically endless; this fact makes polymers useful in a very wide array of applications, such as;
- Thin Films and Sheets
- Elastomers
- Adhesives
- Formed and molded products
- Coatings, paints, and inks
- Yarns and other fibers
and literally thousands more!!
The Physical form of the Polymers result in them existing in a wide range of shapes, sizes, and colors. The following are some distinguishing features:
- Density
- Conditions of heat
- Structure of crystals
- Hardness
- Pulling power
- Machinability
- Formability
- Solubility , etc.
2.3 Application of Polymers.
The purposes for which polymers are employed are yet another way to classify them. Polymers’ versatility stems from their ability to be polymerized into a wide array of materials – tailored specifically to the demands of the application.
The following structural characteristics of a polymer must be considered when attempting to place it into a certain category;
- Strength,
- Elasticity,
- Viscoelasticity
- Anisotropy
2.3.1 Examples of Polymers that are formed or molded;
- Paper and plastic film
- Elastomers Adhesives
- Colors, and inks
- Threads, rovings, and other fiber goods etc.
2.4 Advantages and disadvantages of Polymers;
As is the case with all materials, Polymers have both advantages and disadvantages. Some of the advantages are;
- Cost-effectiveness.
- Greater Chemical resistance than metals.
- No requirement for post-treatment finishing.
- Up to ten times lighter than typical materials
- Significant thermal and electrical insulation properties etc.
Some of the disadvantages are;
- It cannot be easily machined
- Low structural rigidity
- The strength-to-size ratio is less compared to metals.
- Limited heat withstanding capacity etc.
3.0 PLASTICS
Plastics, on the other hand, are a class of Polymers made up of long, branched molecules, or macromolecules.
Plastics are synthetic polymers extracted from petroleum through a polymerization or polycondensation process. Different chemicals and condensation are both used to promote molecular bonding producing Plastics, making them synthetic or semi-organic polymers derived from primarily oil or petroleum. Polymers can be found in nature, but Plastics are wholly synthetic.
Plastics represent a distinct category of polymers, comprised of polymer chains that might be semi-organic or wholly synthetic. While all Plastics fall under the category of Polymers, it’s important to note that very few Plastics qualify as a Polymer.
Broadly stated the most common used Plastics are;
- Acrylic or Polymethyl Methacrylate (PMMA)
- Polycarbonate(PC)
- Polyethylene(PE)
- Polypropylene(PP)
- Polyethylene Terephthalate (PETE or PET)
- Polyvinyl Chloride (PVC)
- Acrylonitrile-Butadiene-Styrene(ABS)
However, due to its polymer composition, plastic shares similar physical characteristics and adaptability to that of Polymers, making it very versatile. There are mainly two types of Plastic: Thermosets Plastics and Thermoplastics.
Thermoset Plastics
Heat hardens thermoset plastics, making the pattern permanent. After being molded, thermosets keep their shape even when reheated. When exposed to high temperatures after setting, thermosets will burn rather than melt. Thermoset polymers are ideal where it is required of , for example, precision components to withstand extreme temperatures without distorting or creeping. Thermoset Plastics too exhibit excellent resistance to corrosion and high temperatures.
Examples of Thermoset Plastics are;
- Polyurethane Epoxy Phenolic
- Some types of polyesters
- Phenolic
- Thermosets have several uses because of their durability and resistance to heat and cold.
Insulators and electronic parts;
- Fire walls
- Mechanical components and protective housings
- Home appliances
- Illumination products
- Electrical Products
Thermoplastics
The molecular structure of thermoplastics is not altered by heating and cooling, unlike that of thermosets. Due to their malleability and ability to melt at high temperatures, thermoplastics are widely used in the fabrication industry. Plastic toys, toothbrushes, bins, and bottles are common examples of consumer goods that fall under this category; they are not subjected to high temperatures.
Thermoplastics can furthermore be either Amorphous or Semi-crystalline, depending on their molecular structure;
- Amorphous Plastics; The polymer chains in amorphous thermoplastics are arranged in a random manner, rather than in a specific pattern. Amorphous thermoplastics are brittle above a certain temperature yet extremely tough when cooled down. Examples of Thermoplastics are; Plastic windows and light fixtures. Both these products benefit from their transparency resulting from the material’s lack of rigidity.
- Semicrystalline Plastics; Polymer strands in a regular pattern, or a crystalline structure with some amorphous regions, characterize semi-crystalline thermoplastics. The plastic’s physical properties are determined by the ratio of its crystalline toa morphous structure. A material’s opacity increases as its crystalline structure grows more complex. When compared to their totally a morphous counterparts, semi-crystalline thermoplastics excel in several areas.
Many different substances can be classified as Thermoplastics , inclusive of;
- PET or Polyethylene
- Simplified polystyrene
- Polypropylene
- PVC, or polyvinyl chloride,
- Polyester Nylon
- TPOs, or thermoplastic olefins, are a class of olefins
- ABS (Acrylonitrile Butadiene Styrene) v Santoprene
- Acetals and many more
Thermoplastics are beneficial in a wide variety of fields and contexts due to the Plastics’ ability to be adapted/modified to its required final form. Some examples are ;
- Injection molding and blow molding products
- A very wide array of retail items
- Many components of motor vehicles
- General products used in the wide array of Engineering applications
- Medical devices
- Containers used for Storing products.
- Supplies for Packaging , and once again many more.
Blow molding fabrication is ideally suited to thermoplastics because of their malleability. This process applies to manufacture bottles, containers, cases, and other hollow parts and components. Blow molding uses compressed air to manipulate the molten plastic resin into a pre-fabricated mold.
SUMMARY
It is clear that the Polymer vs Plastic differentiation is not a simple one , but despite this, there is a Polymer, or a Plastic for practically most requirements and applications.
If in doubt; seek advice from an expert! Gennisi Solutions are able to assist in determining the requirements of the application and provide a solution.


